Behind the Design: How Medtronic Found a Goldilocks Solution to Building an EV-ICD
A behind-the-scenes look at how Medtronic designed its extravascular defibrillator.
December 20, 2023
.png?width=850&auto=webp&quality=95&format=jpg&disable=upscale)
Medtronic's extravascular implantable cardioverter-defibrillator (EV-ICD) may have a mermaid's name, but when the company's R&D team began the project, it was another fictional character that came to mind: Goldilocks.
"When we began the EV-ICD journey, what we were looking for is what I call the 'Goldilocks' solution, or the 'just right' solution that would keep the leads out of the heart and vasculature, but could still provide all of the therapies available in modern transvenous systems such as the ability to pace, the ability to defibrillate at low energy, the ability to keep the device size small, and the ability to retain the longevity of modern systems," Amy Thompson, clinical research strategy director at Medtronic, told MD+DI.
FDA approved Medtronic's Aurora EV-ICD system earlier this year. The system incorporates the company's MRI SureScan technology and the Epsila EV MRI SureScan defibrillation lead. The Aurora EV-ICD system is designed to enable lead placement under the breastbone, outside of the heart and veins.
Thompson recently shared some teachable moments and other takeaways that emerged from the R&D process.
Lesson 1: Make the unfamiliar familiar
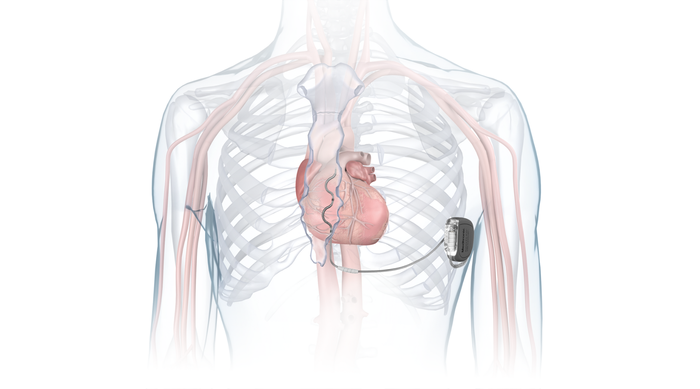
IMAGE COURTESY OF MEDTRONIC
Thompson said one of the biggest challenges of the EV-ICD project was the challenge of belief, which she acknowledged is a common theme when developing any disruptive innovation.
"We were effectively asking our physician partners to think about a new paradigm for defibrillation and a new implant space that may not have been immediately familiar to them," Thompson said. "One story that stands out so vividly for me is one of the very first physicians I ever showed the substernal implant to wrote in his own notes from that day, 'There is a little known space between the heart and the sternum, which the team showed me we can implant within.' And that has, for 11 years, been in my brain."
That moment signaled to Thompson where the research team would need to spend its time on making the unfamiliar familiar, she said. That meant learning and then teaching the anatomy aspect and demystifying the implant procedure to make implanting physicians aware of the capability to implant in that space.
Lesson 2: Disruptive technology requires detailed thinking and a diverse, cross-functional team
To develop the Aurora EV-ICD, Medtronic didn't just need to build a new ICD.
"We needed a new implant procedure; we needed new delivery tools to facilitate that implant procedure; we needed to use imaging in a slightly different way than we had in the past to facilitate that implant procedure; we needed to prove that we could pace, sense, and defibrillate from this new region; in some ways, we needed to retool our pacing engine to provide increased voltage and longer pulse durations; and we needed to think holistically about features that could be important to EV-ICD," Thompson said.
The Aurora EV-ICD system includes features available in the company's transvenous ICDs, and offers additional advantages that are not available with the subcutenous ICD, including:
Anti-tachycardia pacing (ATP), to terminate ventricular arrhythmias (rapid and/or chaotic activity of the heart that can lead to SCA) using low-energy pacing pulses, potentially avoiding a defibrillation shock.
Pause Prevention Pacing, to provide back-up pacing for brief, intermittent, heartbeat pauses.
40 Joule Defibrillation Energy, to deliver life-saving shocks in a device the size of transvenous ICDs (33 cc)
Medtronic exclusive PhysioCurve design, to increase patient comfort and implant acceptance.
11.7-year projected longevity, to reduce device replacement procedures during a patient's lifetime.
Patients who receive the commercially available Aurora device also will benefit from the addition of Smart Sense, an algorithm designed to reduce the potential for inappropriate shocks.
The work didn't stop there, of course. Thompson said the team leveraged its initial clinical implant experience, testing experience, and imaging data to further refine and optimize where the lead is positioned.
Lesson 3: Use disbelief as your fuel
From a mentoring perspective, Thompson said one of the biggest lessons she took away from the EV-ICD project that she intends to teach for the remainder of her career is to use disbelief as your fuel.
"What I mean by that is where people question you or challenge you will become the signals of where you need to spend your time, and where you need to develop the most evidence."
Lesson 4: Bring people along for the ride
The project also showed Thompson the importance of bringing people along with you on the journey. In some cases, for her team, that was the physician partners who became early champions and supporters. Other times it was Medtronic's senior leaders who the team invited into the lab for the earliest look at what they were building and to invite them to be part of the process, which helped to generate excitement at a higher level within the company.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
